With a surging demand for product traceability in China, many companies have participated in the development of traceability systems and the construction of traceability platforms. According to incomplete statistics, thousands of companies have entered into the traceability market, including Neusoft, Inspur, Huawei, JD.com and Tencent. As a national high-tech enterprise, Sheng Ye Information Technology Service (Shenzhen) Co., Ltd. (“SYIT”), a wholly owned subsidiary of Sheng Ye Capital Limited (“SY Capital”, HKEx: 6069), has been leveraging its expertise and track record in medical supply chain management to accelerate the research and development of SaaS systems for the sector, to promote the cooperation of medicine information traceability systems, and strengthen the exchange and sharing of traceability information.
The primary purpose of creating traceability systems is to verify the authenticity of products and establish consumer trust in product quality. In particular, medicine traceability has garnered nationwide attention as medicine is a special commodity crucial to people’s lives and health. Data shows that the global annual transaction volume of counterfeit medicines is between US$75 billion to US$200 billion. In many developing countries in Asia, Africa, and South America, counterfeit medicines account for 10% to 30% of the total sales of medicines, and the illegal trade of counterfeit medicines has taken a death toll of more than 100,000 every year.
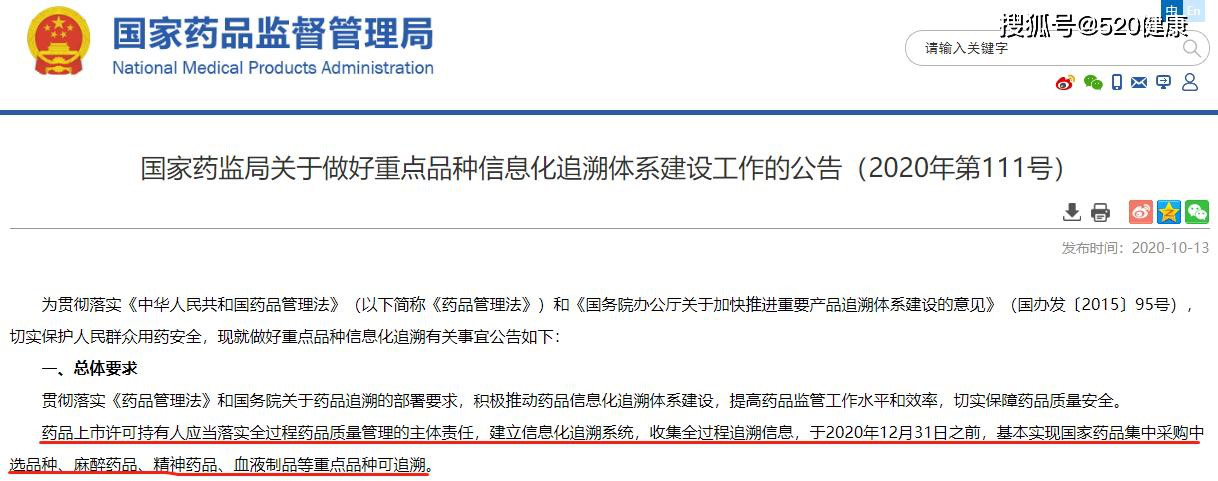
In China, the initiatives to establish medication traceability systems started long ago. In 2006, the former China Food and Drug Administration (“CFDA”), now the National Medical Products Administration (“NMPA”), began to implement medicine electronic supervision and adopted a “medicine electronic supervision code” to track the whole process of drug production and circulation. However, this approach was finally suspended in 2016 due to data security and other issues. In 2018, the NMPA released “the Guidelines for the Construction of Medicine Information Traceability Systems”, which defined the term “medicine information traceability system” as the whole process in which all participants track and trace the different stages of medicine production, circulation and usages by means of information technologies. In 2020, the NMPA issued the “the Notice on the Construction of the Information Traceability System for Key Medicine Products”.
Meanwhile, the NMPC is establishing a medicine traceability cooperation and service platform and continues to improve the traceability data exchange and sharing mechanism. Pharmaceutical businesses and holders of marketing authorization for medicinal products can file their medicine traceability coding rules on the platform, which will help decode the addresses of different medicine information traceability systems. This facilitates the interconnection and data sharing between various traceability systems, thus enabling medicine traceability across the supply chain.
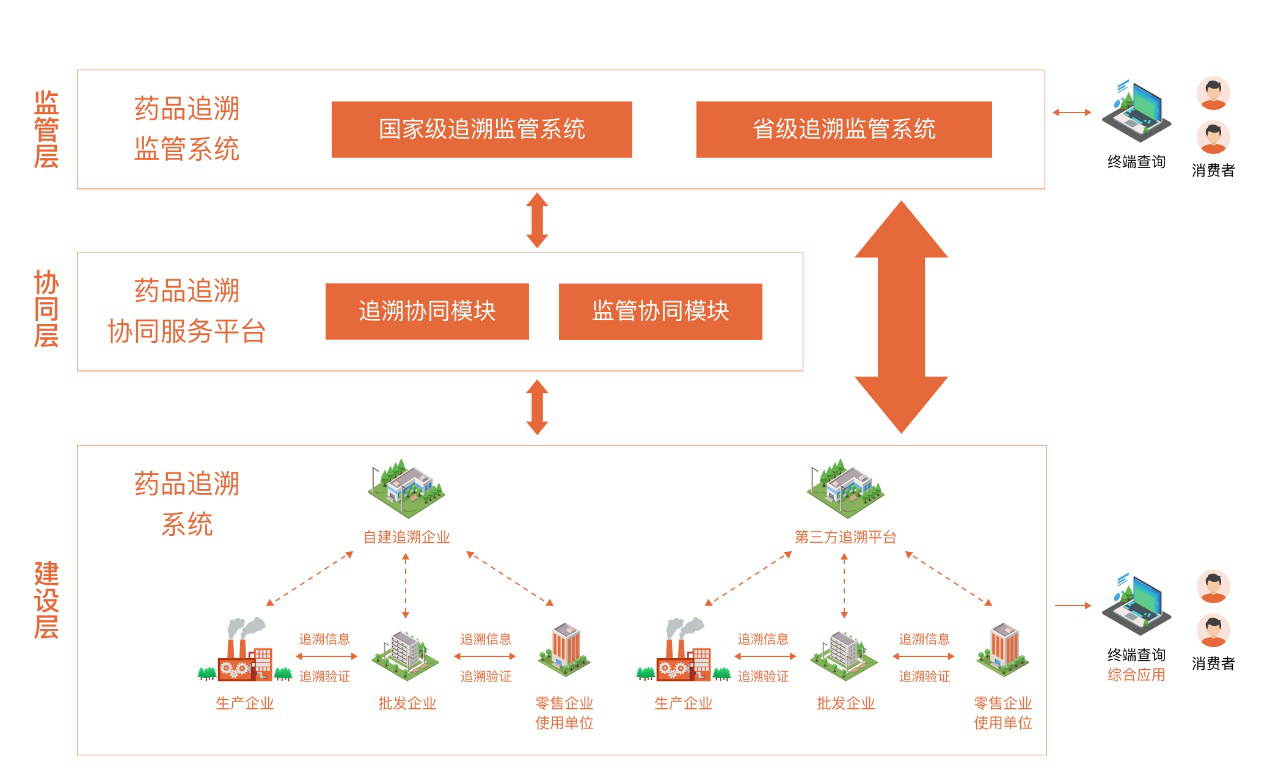
Medicine traceability systems collect the data and keep records of information along the pharmaceutical supply chain, from medicine production, distribution to consumption. They are crucial for tracking down the product sources, checking the whereabouts, and establishing transparency and accountability. These systems aim to strengthen medicine quality, safety management and risk control of the whole process, and are of great importance as it is related to the public health and rights. In addition, medicine traceability systems apply blockchain technologies and distribute the traceability data to the relevant entities based on laws, regulations and legally binding business agreements between these parties. This helps to safeguard data security, establish “data trust”, and ensure that data is properly encrypted, well controllable and shared in real time among various parties in the medicine ecosystem, including manufacturers, distribution companies, sales, consumers, and regulatory authorities. In this way, it solves the fundamental data issue with applying industry data for supervision while enhancing supervision efficiency and quality significantly.
SYIT has comprehensively improved its capabilities in big data technology, industrial application, and operational innovation. Going forward, SYIT will continue to deepen the cooperation with all partners in medicine information traceability systems and construct an innovative ecosystem for the pharmaceutical sector.


